 Back to News
Back to NewsContract Manufacturing: Pharma’s New Growth PillDomestic contract development and manufacturing organisations are raking it in, thanks to India’s emergence as a pharma R&D hub and China’s supply chain issues.
April 05, 2024
AURIGENE PHARMACEUTICAL services, a subsidiary of Dr Reddy’s Laboratories, has lined up big expansion in biologics over next three years. As a start, it is developing a facility for making therapeutic proteins, antibodies and viral vectors (tools to deliver genetic material into cells) at Genome Valley, a biotech park in Hyderabad, to enter contract development and manufacturing of biotech drugs. ‘’It will be operational in first half of 2024. We will provide integrated services right from clinical research to commercial manufacturing for small and large molecules,’’ says Akhil Ravi, CEO, Aurigene Pharmaceutical Services. If Aurigene is set to start as a complete CDMO (contract development and manufacturing organisation), another Hyderabad-based CDMO, Aragen Life Sciences, has bigger plans. It will invest ₹2,000 crore in its Mallapur, Telangana, facility for drug discovery, development and manufacturing for global life sciences industry, says director & CEO Manni Kantipudi.
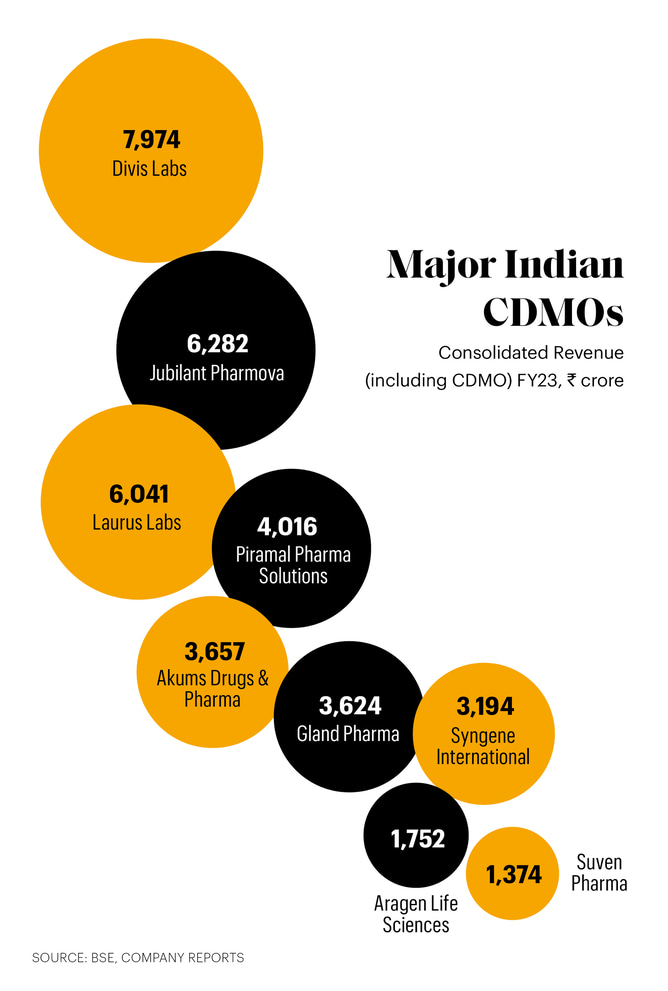
Large established players are also keen to expand their CDMO business. At Kona in Kakinada district of Andhra Pradesh, Divi’s Laboratories is building a factory over 500 acres. Divi’s is one of the largest active pharmaceutical ingredient (API), custom synthesis and intermediates suppliers to global generic makers and innovator multinationals. It reported ₹7,974 crore revenues in FY23 of which 70% came from U.S. and Europe. The new unit will come up by FY25 with Divi’s spending ₹1,200-1,500 crore in first phase, says managing director Murali K. Divi.
Another CDMO player, Laurus Labs, is in the middle of a ₹990 crore expansion. Laurus had started as a contract researcher and manufacturer of APIs before specialising in antiretrovirals, intermediates and formulations. Now, 60% revenue comes from U.S. and European customers. Revenues grew at a compound annual growth rate (CAGR) of 27% in last five years from ₹2,292 crore in FY19 to ₹6,041 crore in FY23. Another CDMO major, Jubilant Pharmova, is expanding in North America — it is spending $370 million in Spokane and Montreal to double sterile injectables capacity over next five years.
These are not all. The list of fast-growing CDMOs working for global MNCs includes Sai Life Sciences, Piramal Pharma Solutions and Supriya Life Sciences as well. The industry is also seeing emergence of contract specialists working for the domestic industry. New Delhi-based Akums Drugs and Pharmaceuticals, which grew sales at a CAGR of 18.7% from FY19 to FY23 (to ₹3,656.6 crore), makes 12.5% of India’s domestic formulations under contract.
The $42 billion domestic pharmaceutical industry has 3,000 companies with 10,500 manufacturing units. Of them, at least 100 are becoming CDMO specialists, an emerging segment that includes API manufacturing and contract research and development. Some are developing biotech drug capabilities too. India’s ability to develop drugs at one-fourth the cost in the West and largest number of US Food and Drug Administration-approved plants outside U.S. is helping companies bag 5-10 year deals from Big Pharma, say experts.
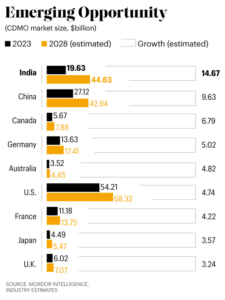
Global CDMO market was valued at $224.6 billion in 2023. Various industry estimates say Indian CDMO market is expected to grow at a CAGR of 14.67% from $19.63 billion in 2023 to $44.63 billion by 2029 by tapping API and contract research opportunities from many new molecules going off-patent in coming years. Global CDMO growth is projected at 6-7% over next five-six years. Let’s delve into opportunities that will help government in this ‘Make in India and Export’ initiative.
Covid/China Factor
One of the biggest growth triggers for players such as Divi’s Lab was Covid. Supply of key vaccine raw materials took Divi’s revenues from ₹6,861 crore in FY21 to ₹8,991 crore in FY22. Vaccine makers such as Serum Institute, Biological E and Bharat Biotech became poster boys of India’s vaccine capabilities, helping them get more exposure and acceptance globally. “Our CDMO business was zero a couple of years ago. Now, there is revenue visibility of ₹200 crore over two-three years, with two contracts signed and discussions on with a couple of European companies,’’ says Saloni Satish Wagh, director, Supriya Life Science, which specialises in APIs for anaesthetics, anti-diabetes and anti-depression drugs. It reported ₹460 crore revenue in FY23.
The momentum is continuing. Integrated established player Piramal Pharma got over 40% more orders in H1FY24 compared to H1FY23. “During the quarter we received our first integrated antibody-drug conjugate order involving monoclonal antibodies,” says Nandini Piramal, chairperson, Piramal Pharma. “We continue to build on our improved performance in FY24 with 14% YoY revenue growth in Q3 along with a significant improvement in EBITDA margin. Our CDMO business is delivering healthy growth with robust order inflows, especially for differentiated offerings and innovation related work,” she says. Piramal’s CDMO business grew 7% to ₹4,016 crore in FY23.
Biocon’s Syngene, which grew revenue from ₹1,485 crore in FY18 to ₹3,264 crore in FY23, says business is gaining momentum after Covid. “All business divisions delivered growth through the year (FY23), triggering investment in additional laboratory capacity and new facilities at our campuses in Bangalore and Hyderabad, as well as creating over 1,000 new jobs.” says Jonathan Hunt, managing director and chief executive officer, Syngene International.
Even established formulation companies having long relationship with global innovators are developing API and CDMO units. It is a small pie of their business though. India’s largest pharmaceutical company, Sun Pharma, which offers contract research and manufacturing services, got 5% of its ₹43,300 crore sales from API and other pharmaceutical solutions in FY23. Cipla, once one of the largest suppliers of APIs to global firms, got 4% of its ₹22,753 crore business (FY23) from API and pharmaceutical technology solutions. Other examples of formulators floating CDMO subsidiaries include Aurigene. ‘’Globally, only a handful of organisations possess the complete set of skills and tools at scale necessary to take a scientific idea all the way from early research to patient trials. We took the decision some years ago to forward integrate, first into development services and then into manufacturing,’’ says Jonathan Hunt of Syngene International.
Thanks to these mega plans, India is set to beat China and become a major player in the global CDMO market, projected to grow from $238.47 billion in 2024 to $330.36 billion by 2028. U.S. is projected to go from $54.21 billion in 2023 to $68.32 billion by 2028 by growing at a CAGR of 4.74%. The next biggest is China, worth $27.12 billion in 2023, and poised to grow at 9.63% CAGR to $42.94 billion by 2028.
Catching Up With China
India’s rise is expected to come at the cost of China, which got a foothold in the CDMO market thanks to its robust API or bulk drug supply chain, huge facilities and drug development capabilities. Liberal government funding and 25-30% cheaper labour also made it a favourite contract manufacturing destination.
However, the Chinese faced problems after Trump administration imposed trade sanctions and Covid disrupted drug supply chains. ‘”Most multinational pharma players were heavily reliant on China for making drugs on contract. They started de-risking by including Indian suppliers,” says a veteran industry analyst. Another major advantage for India was its large population of English-speaking professionals.
‘Supply chain diversification (from Europe, U.S. and China), increase in strategic and PE investments in world-class infrastructure, availability of skilled talent and management attention have led to a positive outlook for the CDMO space,” says Akhil Ravi of Aurigene Pharmaceutical Services. He says digitisation of manufacturing, systems to ensure end-to-end connectivity and automation of track and trace solutions have made global pharmaceutical and biotech companies choose India as a hub for R&D and manufacturing.
Policy Push
The boom has not come about in a vacuum. Experts say until India joined the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights in 2005, Big Pharma was sceptical about transferring valuable intellectual property rights to Indian drug manufacturers despite many Indian companies matching Chinese capabilities.
Funding, too, was an issue. That is changing as government has come out with several schemes to promote ‘Make in India.’ Prime among them is Production Linked Incentive (PLI) Scheme for pharmaceuticals, launched in 2021 with an initial outlay of ₹15,000 crore over six years. It’s expected to attract investments of ₹17,425 crore. Also, PLI Scheme for bulk drugs, with an outlay of ₹6,940 crore, will boost production of 41 critical bulk drugs. Many companies started building bulk drug parks in India after government allowed 100% FDI in the sector. Cumulative FDI equity inflow in drugs and pharmaceuticals industry was $21.46 billion between April 2000 and March 2023, according to an IBEF report.
“Schemes like PLI and bulk drug parks can reduce dependence on China by 25-30% in four-five years,’’ says Deepak Jotwani, assistant vice president and sector head, Corporate Ratings, ICRA. India imports about ₹35,000 crore worth of bulk drugs, about 35% of its requirement, in a year. Self-reliance in bulk drugs is crucial for building CDMO capabilities.
Consolidation On Cards
Fast growth means CDMOs are finding it easy to get backing of PE funds and professional managers. Prime among them are former Ranbaxy CEO D.S. Brar-led Aragen and Ajay Piramal-led Piramal Pharma. Sources say in May 2021, Goldman Sachs acquired about 33% in Aragen for ₹2,400 crore, valuing the company at $1 billion. Aragen’s previous investors included Chrys Capital and Sequoia Capital. Piramal’s pharma business, which grew from ₹1,537 crore in FY11 (after sale of formulations division to Abbott) to ₹5,419 crore in FY20, sold a 20% stake to US-based Carlyle for ₹3,700 crore in May 2020. Piramal invested in CDMOs Hemmo Pharmaceuticals ($106 million), Yapan Bio and Allergan (JV).
But the biggest deal in India’s CDMO market happened in December 2022 when PE firm Advent International acquired Hyderabad-based Suven Pharmaceuticals, which was demerged from its parent entity Suven Life Sciences in 2020, for over ₹9,500 crore. Venkateswarlu Jasti and family-promoted Suven Pharma had become one of the leaders in the CDMO space with high growth (20% CAGR over last four years) and profitability (43% EBITDA margins). Advent merged Suven with its Cohance Lifesciences to create a new identity for its CDMO and API platforms. Sources also point at several small and medium deals for adding complex therapies, next-generation biological capabilities and manufacturing plants. A few months ago, half a dozen global funds were in talks to acquire Hyderabad-based Sai Life Sciences, another major privately held pure-play CDMO business, say news reports. TPG Capital already holds nearly 43.3% in the company.
Bio-Focus And Strategic Deals
Of late, Indian companies have also been either acquiring or partnering with other companies to add capabilities, especially in biotech manufacturing. Early January, Cipla partnered with biopharmaceutical CDMO Kemwell Biopharma and Manipal Group to start a joint venture in U.S. to develop novel cell therapy products. Cipla and Kemwell Biopharma already have a JV in U.S., Aspergen, which was formed in 2022 to develop biosimilars for global markets. The JV is developing two projects. ‘’Kemwell has built a world-class facility in Bangalore and made India an emerging hub for cell therapy development and manufacturing,” says Anurag Bagaria, chairman and CEO, Kemwell.
In December, Syngene International acquired a multi-modal biologics manufacturing facility from Stelis Bio-pharma for ₹617 crore to add 20,000 litres of biologics drug substance capacity and a high speed, commercial scale, fill-finish unit. Syngene has also acquired 17 acres to expand operations in Genome Valley, Hyderabad.
Nandini Piramal says Piramal Pharma Solutions continues to see good demand in niche areas. “New capabilities and capacity expansion at Riverview (U.S.), peptide facility at Turbhe in Maharashtra and Ahmedabad are witnessing healthy demand,” she says. The integrated global CDMO has four facilities in U.S., two in Europe and nine in India and offers end-to-end development and manufacturing solutions. It supplies to over 100 countries.
Aurigene is spending $40 million to build a unit for therapeutic proteins, antibodies and viral vectors. Aragen is setting up a $30 million facility in Bangalore. Aurobindo’s CuraTeQ Biologics is entering contract manufacturing operations for biologicals and building a ₹300 crore facility. Its Auro Vaccines is already working with global vaccine developers.
Sources say while India has made impressive strides, it has a long way to go as it lacks capabilities and an ecosystem in large molecules or biotech drug development. It takes at least three-five years for big pharma players for evaluation of facilities, work culture and manufacturing systems before they award a major contract to companies in India.
While these are temporary hiccups, India is likely to surge ahead in CDMO space on the back of massive investments and entry of professional agencies.
Source: Fortune India
