

Study animal: C57BL/6
Route of administration: bleomycin injections subcutaneously on both flanks
On study termination: Terminal blood for serum, harvest of skin patches for histology and hydroxyproline, harvest lungs, BAL and BALF
Induction of the Scleroderma is confirmed as bleomycin administration results in decrease in the body weight (Fig.1) in dose dependent manner and increase in the lung weight (Fig. 2). Body weight decreases because bleomycin instillation causes decrease in both soleus and visceral epididymal fat masses whereas the increase in lung weight is due to increase of the pulmonary tissue density. Bleomycin administration also increases the Leukocute count in BAL (Fig. 3) and elevates soluble collagen content in BALF as assessed by a Sircol collagen dye binding assay (Fig. 4). Increase in tissue hydroxyproline content also confirms the induction of SSc.
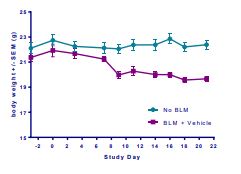
Fig.1

Fig.2
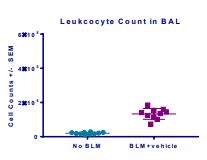
Fig.3

Fig.4
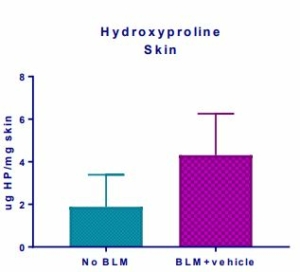
Fig.5
Trichrome (MT) staining and Alpha Smooth Muscle Actin (αSMA) Immunohistochemistry was performed for histology analysis and to confirm the fibrosis of the skin.
Histopathology scores demonstrated hyperplasia of epidermis, necrosis of epidermis and dermis, inflammation of epidermis and dermis and vascular necrosis (Fig. 6) whereas MT staining demonstrated increased mean dermal thickness in bleomycin injected animals (Fig. 7). In samples from BLM mice, αSMA-immunolabeled spindle cells and matrix are abundant in the deep dermis, corresponding with areas of inflammation and spindle cell proliferation seen with hematoxylin and eosin (H&E) stain (Fig. 8, Fig. 9).

Fig.6
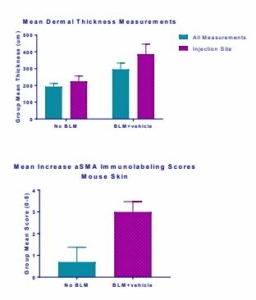
Fig.7 Fig.8
The results also demonstrated that bleomycin administration resulted in the pulmonary fibrosis in the test animals. Pulmonary fibrosis was characterized by expansion of alveolar walls and rare formation of nodular masses by variably dense fibrous connective tissue (collagen), primarily in subpleural and perivascular locations (Fig. 10) (Fig. 11). Results from the MT staining identified collagen, ranged from thin, pale blue fibres to dense, intense blue bands of fibrous tissue (Fig. 12).

Fig.9
Cell infiltration scores demonstrate infiltrates and aggregates of mixed inflammatory cells, including lymphocytes, neutrophils, macrophages, and plasma cells, in areas of interstitial expansion, with abundant lymphocyte
aggregates in perivascular/peribronchiolar zones (Fig. 13). In areas of fibrosis, alveoli rarely contained foamy alveolar macrophages or were lined by hypertrophied and hyperplastic type II pneumocytes.

Fig.10 | Fig.11

Fig.12
Aragen has successfully developed the bleomycin- induced Scleroderma mouse model that accurately demonstrates the pathophysiology of the disease along with all the symptoms as seen in the affected human patients. These models provide an excellent platform to access the pre-clinical efficacy of novel anti scleroderma molecules and to study the disease pathophysiology.
To know more about Aragen’s capabilities and different animal models write to us on bd@aragen.com.
