

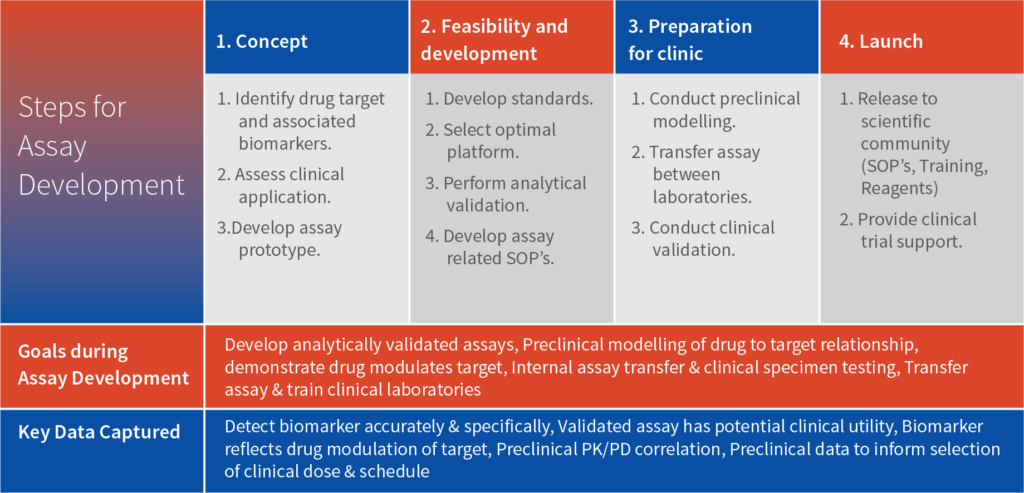
Intox provides a range of potential pharmacodynamic (PD) biomarkers that can be considered for establishing biosimilarity of the molecules. Below is the list categorized by therapeutic area:
It is crucial to emphasize that when choosing PD biomarkers for biosimilars, careful consideration should be given to the specific therapeutic area, disease indication, and the clinical endpoints established for the reference biologic. Regulatory authorities commonly offer guidance regarding the selection and validation of PD biomarkers to facilitate the demonstration of biosimilarity.
1. Single-dose PK: This study assesses the PK profile of the biosimilar and the reference biologic after a single administration. It measures drug concentration levels in blood or other relevant tissues over time to determine the drug’s absorption, distribution, metabolism, and elimination characteristics.
2. Multiple-dose PK: This study evaluates the PK profile of the biosimilar and the reference biologic after multiple administrations. It assesses drug accumulation, steady-state concentrations, and any potential drug interactions.
3. Bioavailability and bioequivalence: These studies compare the bioavailability of the biosimilar with the reference biologic to ensure similar systemic exposure. Bioequivalence studies establish that the biosimilar and reference product have comparable PK profiles.
4. Immunogenicity: PK studies also include assessments of immunogenicity, which measure the immune response to the biosimilar and the reference biologic. These studies determine the presence of anti-drug antibodies (ADAs) and their potential impact on PK parameters.
1. Biological activity: PD studies evaluate the similarity of the biosimilar’s biological effect compared to the reference biologic. They assess the drug’s target engagement, downstream effects on relevant pathways, and relevant biomarkers associated with the therapeutic mechanism of action.
2. Clinical endpoints: Depending on the therapeutic area, clinical endpoints specific to the disease indication may be measured. These endpoints could include disease-specific measures, symptom improvement, or overall patient outcomes.
1. Switching studies assess the impact of transitioning patients from the reference biologic to the biosimilar or vice versa. They evaluate whether any differences in PK, PD, safety, or immunogenicity arise when patients switch between the two products.
2. Interchangeability studies go a step further by determining if the biosimilar can be used interchangeably with the reference biologic without compromising safety or efficacy. These studies are conducted according to regulatory guidelines specific to each jurisdiction.
Few biosimilar approvals have relied on clinical pharmacology studies, comparing PK and PD data, rather than conducting large clinical trials with efficacy endpoints. These approved biosimilars utilized established and sensitive PD biomarkers that are closely linked to the reference product’s known pharmacology. These biomarkers have demonstrated a strong correlation or acted as surrogates for clinical outcomes. However, it is not mandatory for a PD biomarker in biosimilar development to have a direct association with clinical outcomes.
The use of PD biomarkers in biosimilar development aims to establish similarity rather than independently verify the safety and effectiveness of a biosimilar product. Therefore, the considerations for PD biomarkers used to support biosimilarity differ from those for new drug approvals. While having a correlation between the PD biomarker and clinical outcomes can be advantageous, it is not mandatory. PD biomarkers that reflect the biological product’s mechanism of action offer heightened sensitivity in detecting meaningful differences between two products. This opens doors for biomarkers that were previously secondary or exploratory endpoints to assume crucial roles in biosimilar development programs. Additionally, if information on a suitable PD biomarker is lacking, there is an opportunity to discover new PD biomarkers using innovative methodologies.
