
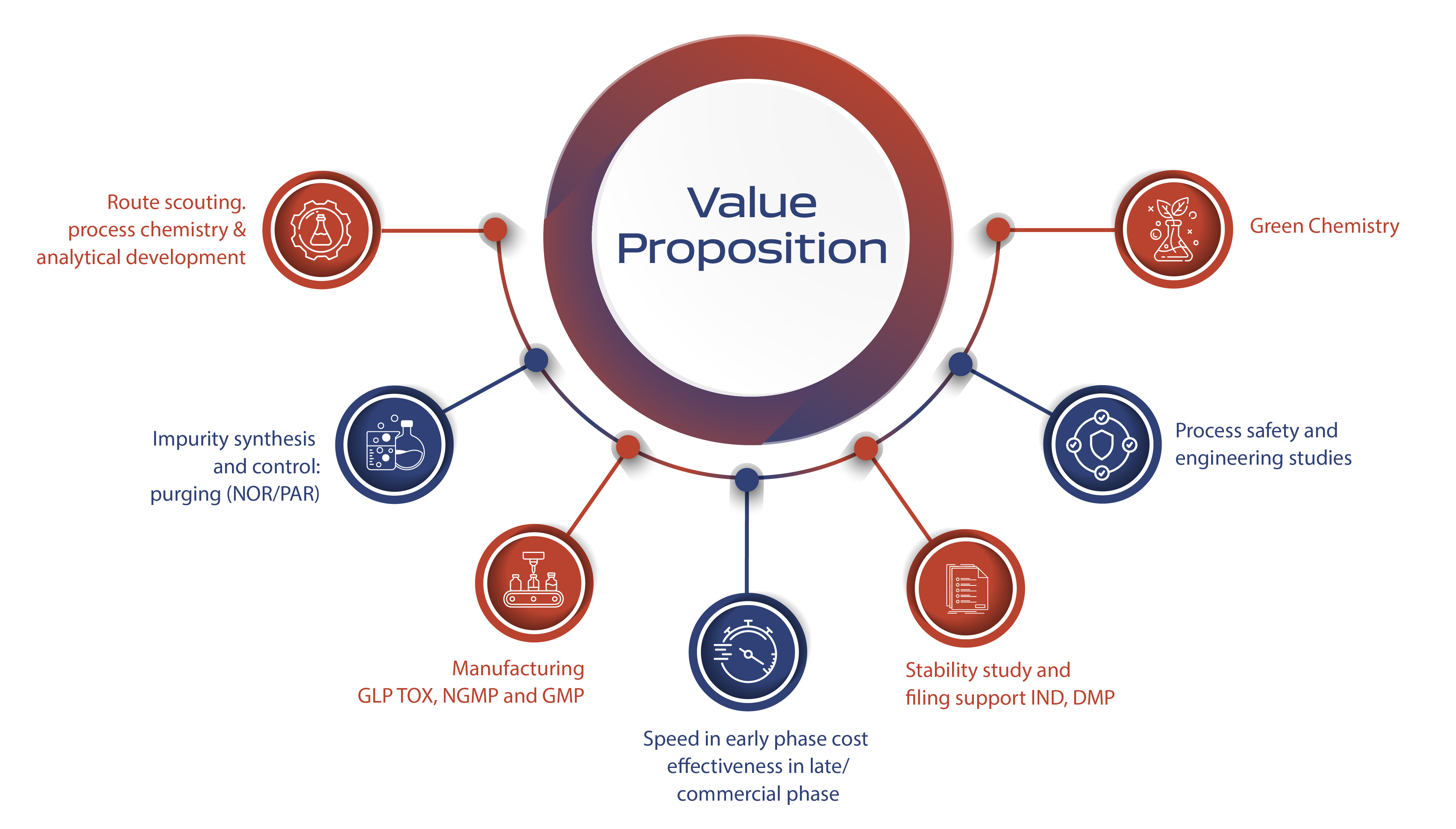
Our value proposition lies in our experienced and talented team, adept at managing cross-functional transitions effectively. This expertise facilitates efficient and swift project progression from pre-IND to NDA and beyond through strong cross-functional linkages. Our development philosophy emphasizes phase-appropriate development, prioritizing speed in early phases, and early evaluation of regulatory, safety, and engineering aspects. We are committed to creating safe, robust, and scalable processes with a constant focus on sustainable and green opportunities.
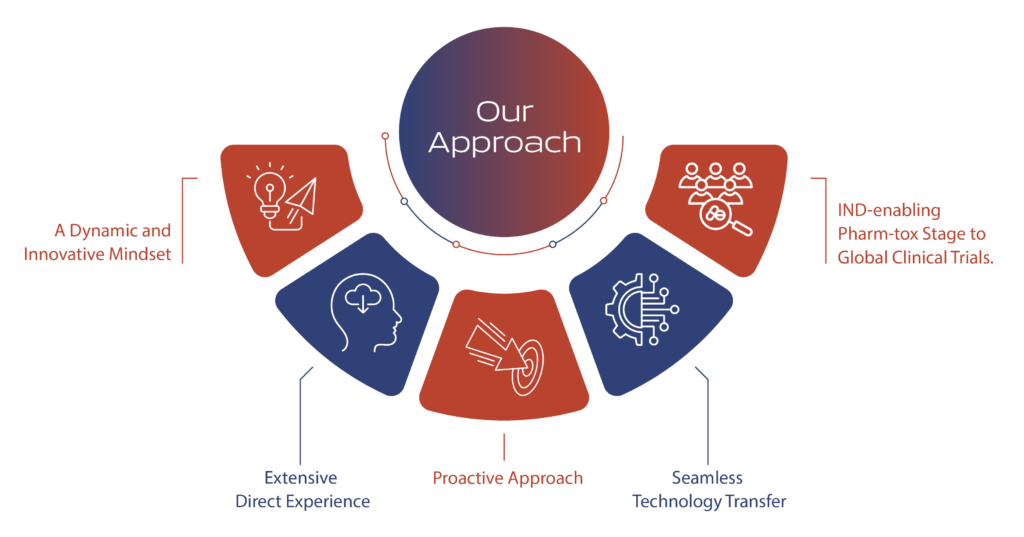
Seamless integration across multiple functions, including Environmental Health and Safety (EHS), project management, quality control, quality assurance, regulatory affairs, and supply chain management, is at the core of our operational excellence. Our commitment is to meet and exceed customer expectations on quality, quantity, and on-time supply of drug substances.
