

The rapidly evolving biologics industry is driven by the need for higher productivity, cost efficiency, and reduced facility footprints. Traditional fed-batch processes have reliably produced therapeutic proteins but face limitations in meeting growing market demands. Commonly explored intensification strategies include N-1 perfusion, full perfusion at production scale, and concentrated fed-batch processes. While each offers advantages, they also come with increased complexity, capital investment, or marginal gains.
In response, we developed an integrated upstream intensification strategy that combined high cell density cell banks, optimized seed train perfusion, and a fortified concentrated fed-batch process. This approach synergized the strengths of various intensification methods while minimizing their drawbacks, delivering significant productivity improvements, and maintaining product quality.
Industry-standard approaches to intensification typically involve trade-offs. Traditional fed-batch processes offer moderate titers (~4–6 g/L) and monthly outputs around 40-50 kg using 4 x 2kL reactors. Full-scale perfusion production boosts productivity further but demands substantial capital investment and staffing resources. N-1 perfusion can shorten seed train duration and modestly increase titer, but it introduces a slightly high media consumption and increases operational complexity. Concentrated fed-batch processes improve titers but require more media than N-1 perfusion (Figure 1). Each approach offered incremental gains—but also came with trade-offs in cost, complexity, or scale-up risk. So, the choice of intensification is very important to get a meaningful outcome.
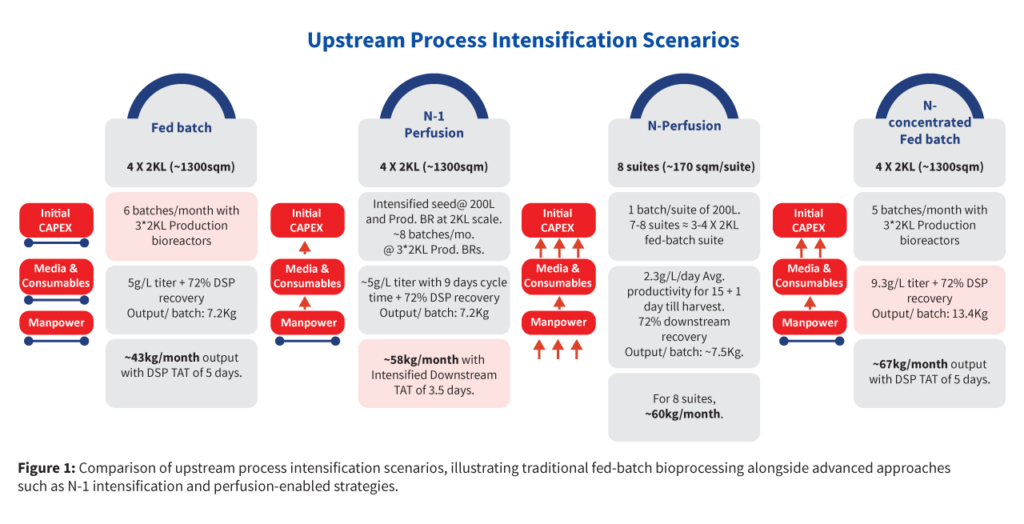
The challenge was to design an upstream intensification platform that achieves the productivity benefits of advanced perfusion systems without excessive complexity or capital expenditure, while ensuring appropriate product quality.
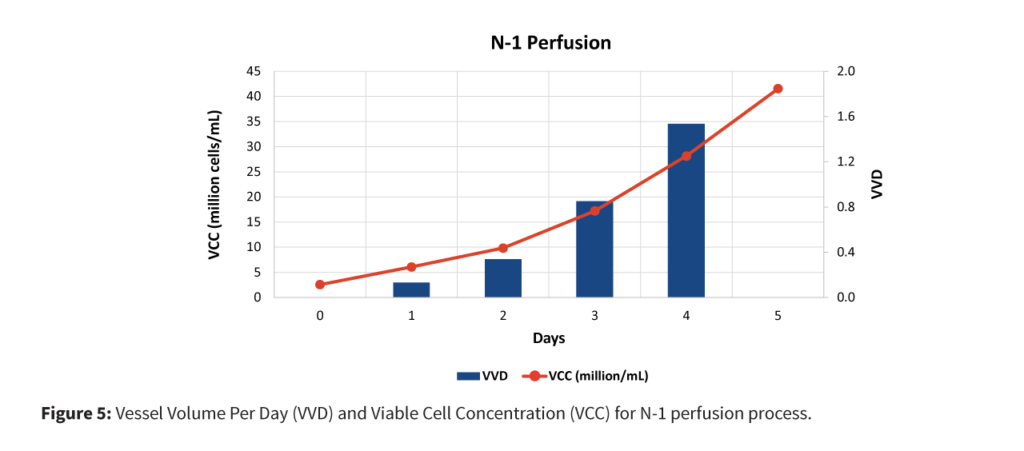
To accelerate the overall bioprocess timeline while maintaining cell quality and productivity, a streamlined and intensified seed train and production strategy was implemented. The process (Figure 2) began with the CSPR screening at shake flaks to identify best condition to achieve a higher viable cell density at shake flask, establishment of high cell density cell banks (30–70 million cells/mL) to provide a robust foundation for seed expansion. Subsequently, N-1 perfusion was performed using a perfusion device, enabling rapid expansion of viable cell numbers. Upon reaching the desired cell density, N stage production-scale bioreactor was directly inoculated from the N-1 stage, bypassing intermediate bioreactor steps to streamline the workflow.
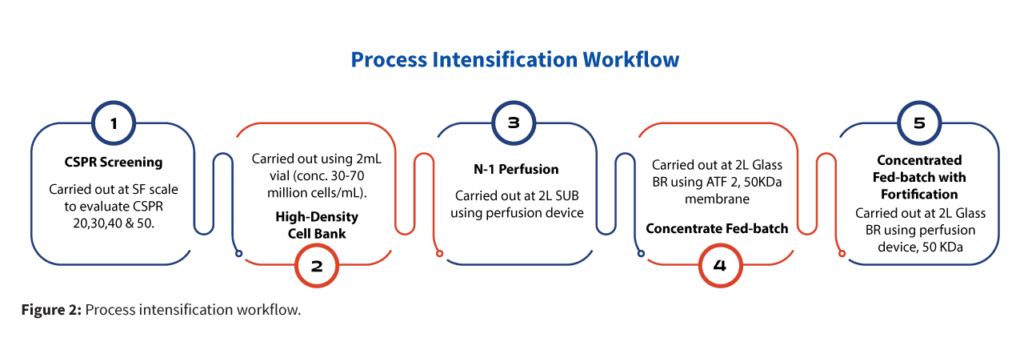
To further optimize scale-up, traditional intermediate-scale bioreactors could be replaced with perfusion-enabled Wave bioreactors at the seed stage, simplifying process logistics and reduce Cost of Goods Sold (COGS).
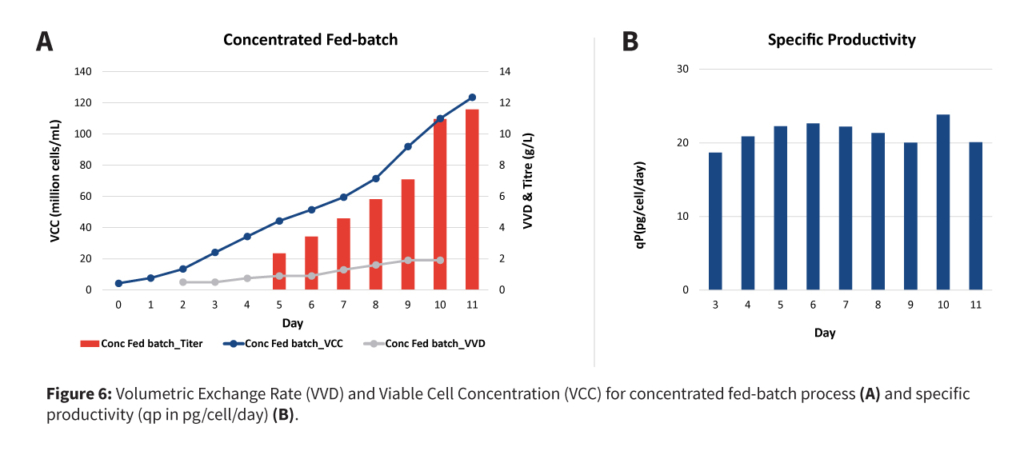
At Aragen, for process intensification, a concentrated fed-batch approach has been chosen to reduce the operation complexity and betterment of COGS. In this process a 50 kDa hollow fibre membrane has been used to retain monoclonal antibodies and allowing only waste metabolites to be removed from the system while ensuing the continuous addition of fresh nutrients.
Fresh media addition rate was adjusted in such a way that it helps to increases the cell mass. The primary objective was to get a higher a viable cell density (VCD) of > 100 million cells/mL in a short period. Once the viable cell density reaches >100 million cells/mL, 75% culture is harvested and purified up to Protein A, followed by LPT and intermediate depth filtration steps. At this stage, spent media analysis should also be performed to understand the key media component depletion trends of key media components. At Aragen, a robust spent media analysis method has been developed, which can identify 85 components divided into seven categories: amino acid, acids, vitamins, amino acid derivatives, nucleotides, nucleosides, choline-related components, and others.
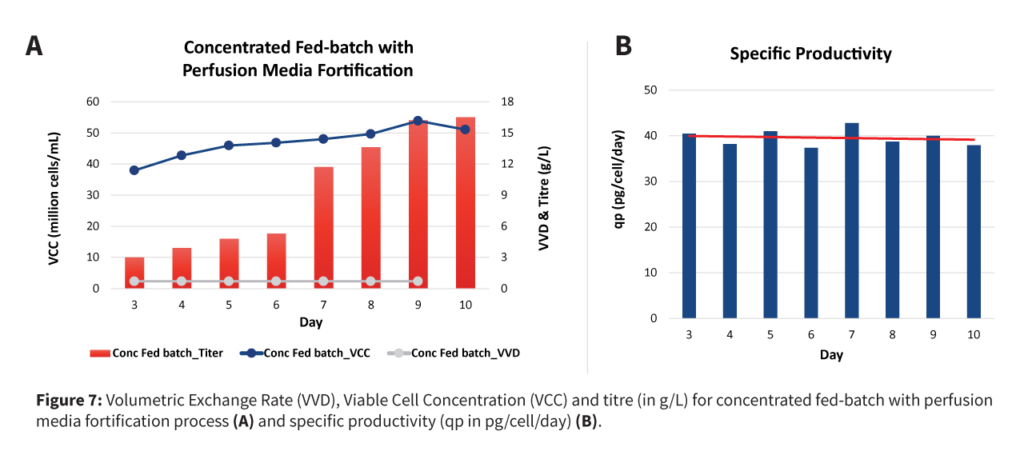
A fresh media top-up will then be performed with the remaining 25% culture to achieve the final VCD in a range of 25-30 million cells/mL. The perfusion rate will be adjusted to maintain a VCD between 50-60 million cells/mL, providing greater operational flexibility using a single-use bioreactor. At this stage, spent medium analysis data should be leveraged to fortify the media with required amino acids, reducing media consumption while maintaining optimum nutrient levels.
The intensification strategy combined high cell density cell banks, N-1 perfusion, concentrated fed-batch, and targeted media fortification to enhance productivity and efficiency without compromising product quality or increasing complexity.
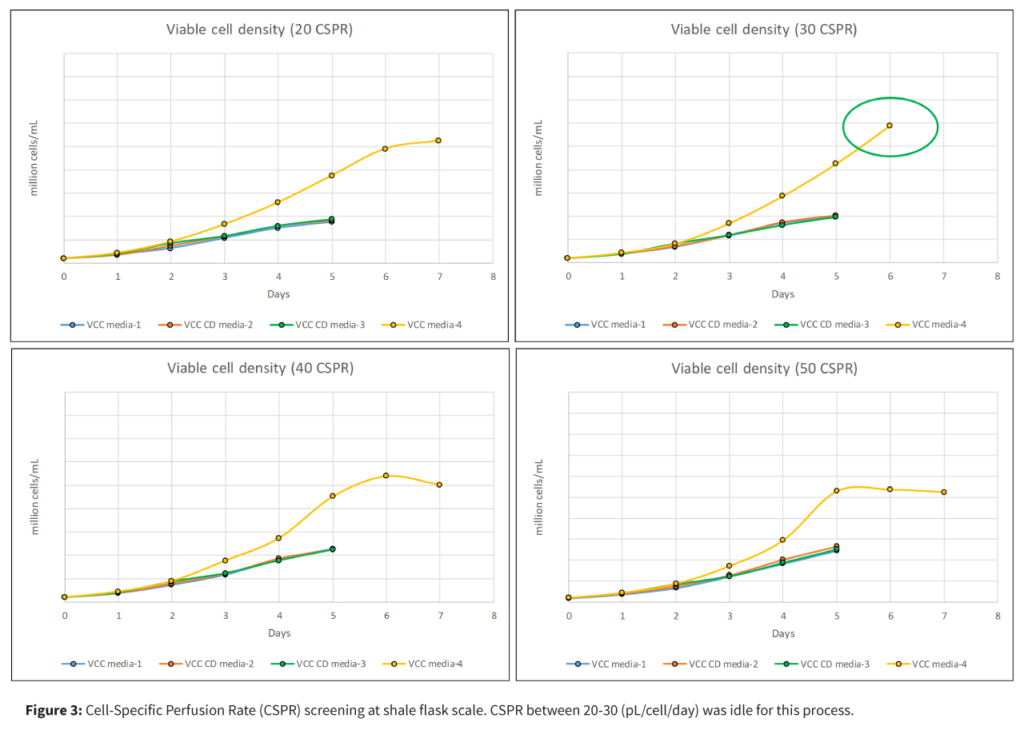
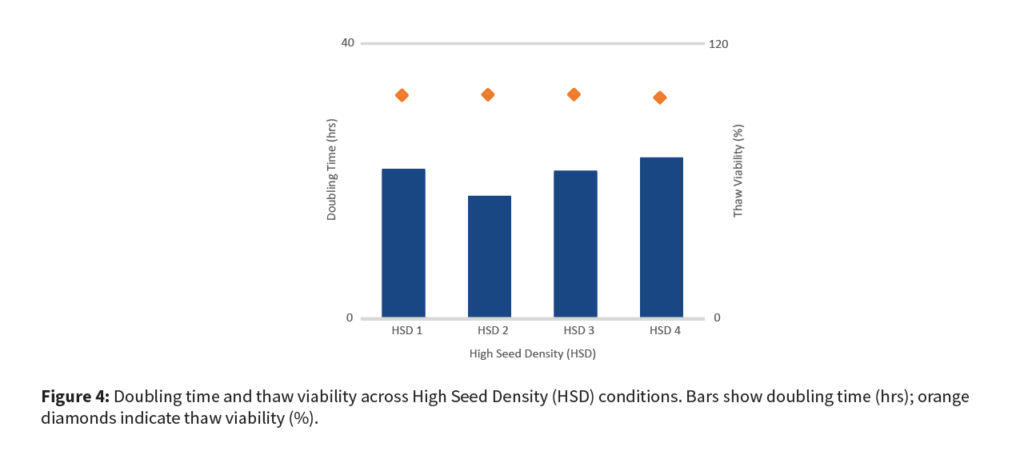
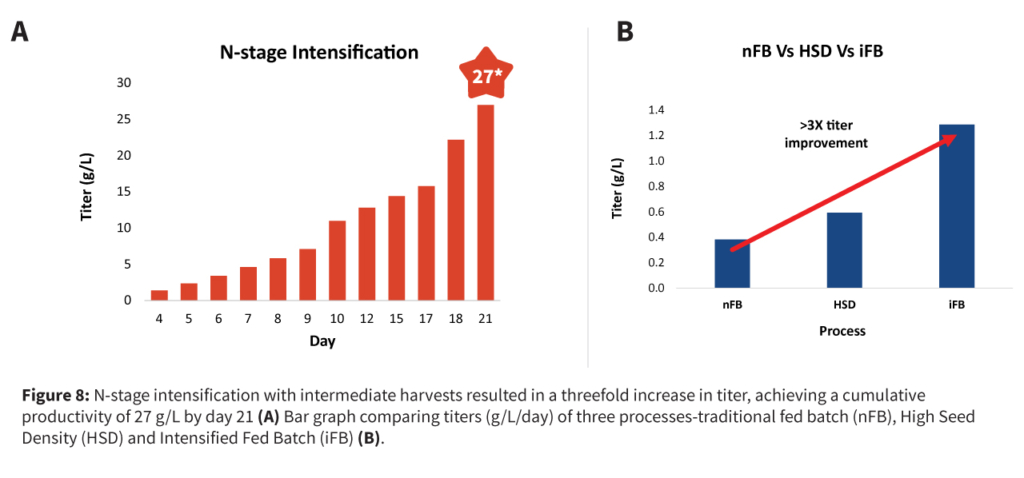
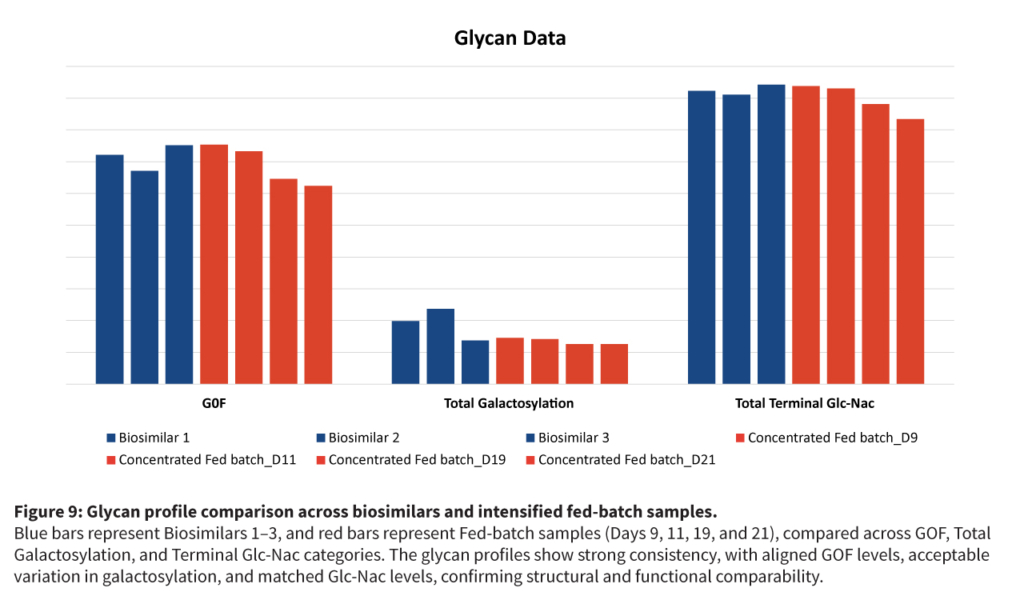
This approach delivered a threefold increase in titer and production capacity while maintaining cell viability and product quality, all without the higher costs of full perfusion systems.
By integrating high-density cell banking, optimized perfusion seed trains, and fortified concentrated fed-batch culture, our approach takes upstream process intensification beyond traditional methods. This strategy allows biologics manufacturers to achieve much higher product titres and monthly output, all while keeping complexity and costs manageable.
Our results demonstrate a practical and scalable pathway for next-generation biologics manufacturing—one that balances productivity, quality, and cost efficiency. Importantly, this approach can be implemented without large-scale perfusion facilities,
helping manufacturers meet growing global demand, use resources more efficiently, and maintain regulatory compliance.

