

Custom Peptide Synthesis — Versatile Chemistries, Faster Progress
PeptARx peptide discovery platform delivers bespoke peptide design and synthesis across modalities, lengths, and complexities—optimized for your discovery timelines and downstream development.
What We Synthesize
Linear Peptide Synthesis
Modified Peptide Synthesis (Discovery Grade Customization)
PeptARx offers a full spectrum of peptide modification strategies to optimize stability, functionality, and performance for your program:
Beyond side-chain and backbone customization, we seamlessly integrate unnatural amino acids into sequences—expanding chemical space and unlocking new therapeutic possibilities.
For more information on our catalogue of unnatural amino acid products (500+), please CONTACT US.
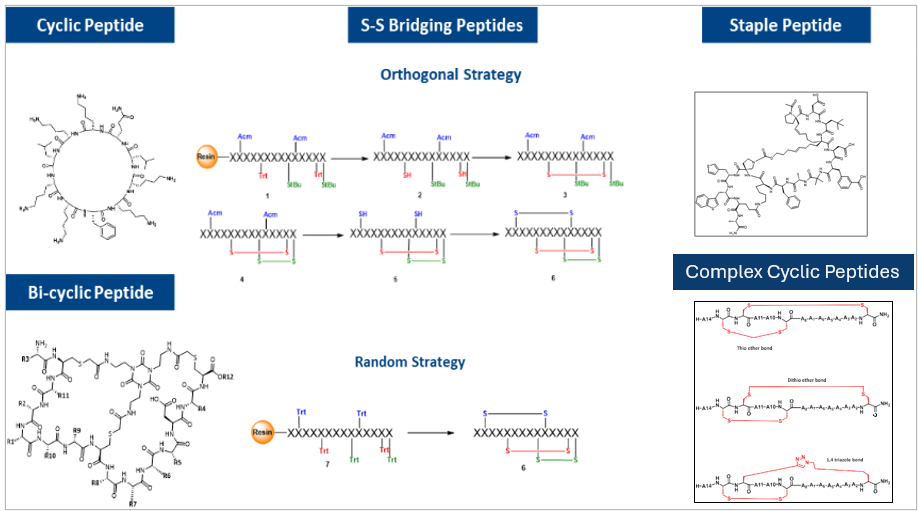
Cyclic & Macrocyclic Peptide Expertise
Cyclization is a proven approach to improve peptide potency, stability, and target engagement. PeptARx, Aragen’s integrated peptide platform, offers end-to-end capabilities for cyclic and macrocyclic peptides—covering molecular design, synthesis, analytical characterization, and scale-up to advance complex peptide programs.
With deep expertise in macrocyclization, delivering more than 2,500 cyclic peptides annually across a wide range of structural complexities and design requirements.
Aragen’s Multi‑Chemistry Cyclic Peptides with Diverse Conjugations
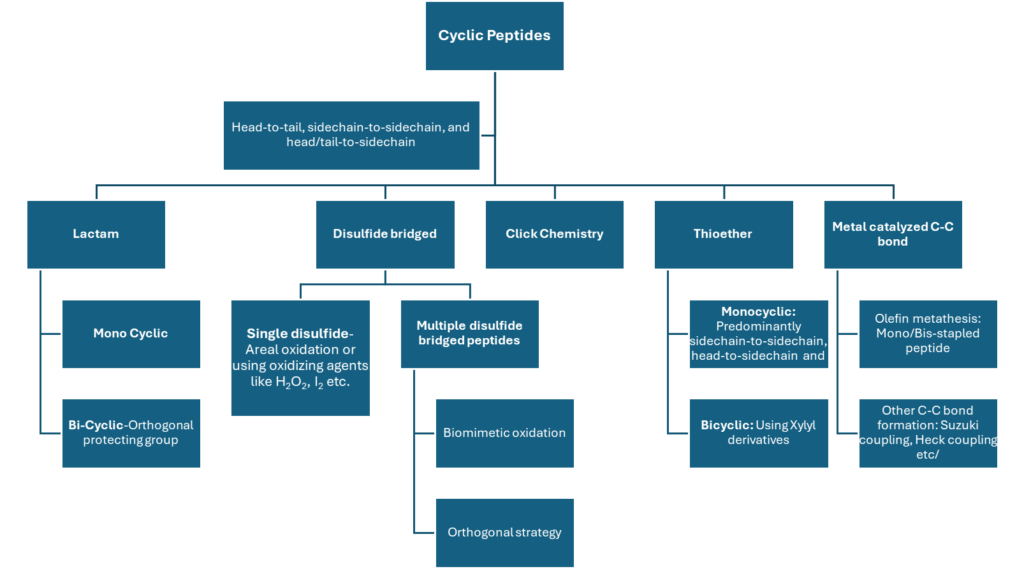
Cyclic Peptides Synthesized at Aragen
Library Synthesis & Parallelization (High-Throughput Discovery)
Coupled with robust analytical workflows, we consistently deliver peptides at exceptional purity—up to 99.9%—meeting stringent research and development standards.
Backed by extensive experience, our team has successfully produced [1500+] linear peptides and [2500+] cyclic peptides, along with numerous complex modifications, over recent years.
Advanced Peptide Synthesis
We support the following advanced peptides.
Specialized Reactions on Solid Support

