Comparative Incidence of Common Spontaneous Neoplastic Findings
Tables 3 and 4 present the comparative incidence of common spontaneous neoplastic findings at Intox, which in general are seen to be comparable to the published databases. It may be noted that the comparison could not be made using any statistical tools in absence of data from individual studies. Certain important trends in tumor incidence, such as the most common spontaneous tumors, tissues/organs with higher and lower incidence of tumors, and the types of tumors, were found to be similar in the Intox’s study and the two published databases. This was despite the smaller number of mice tested in the study of Intox.
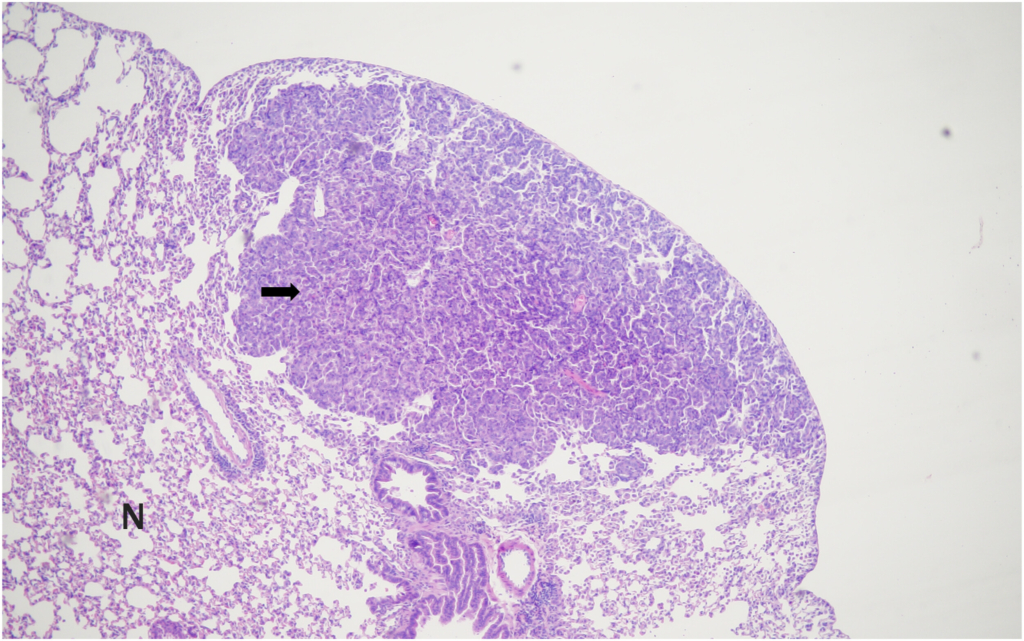
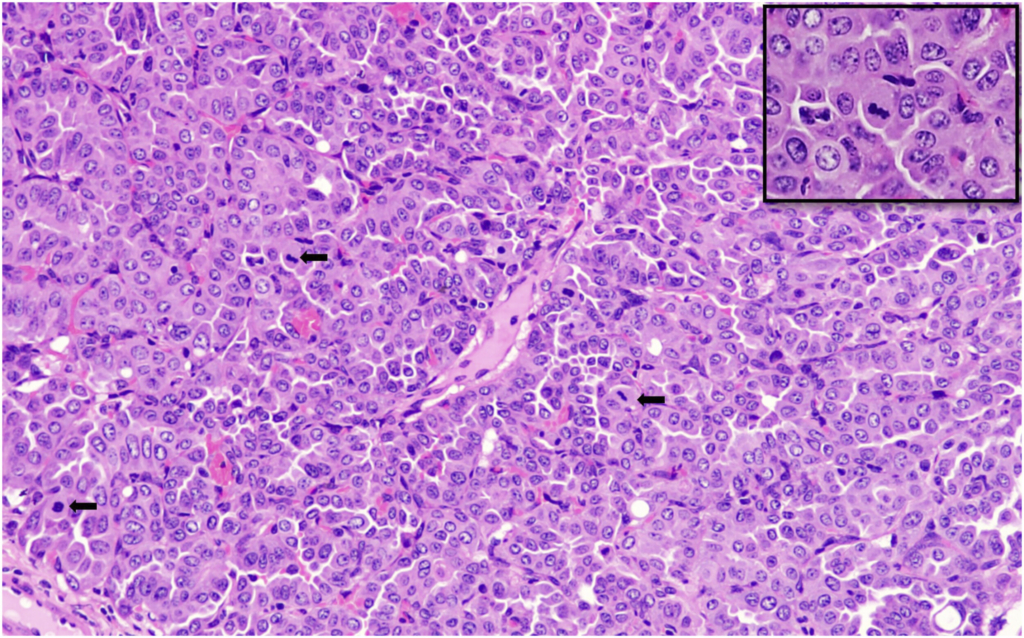
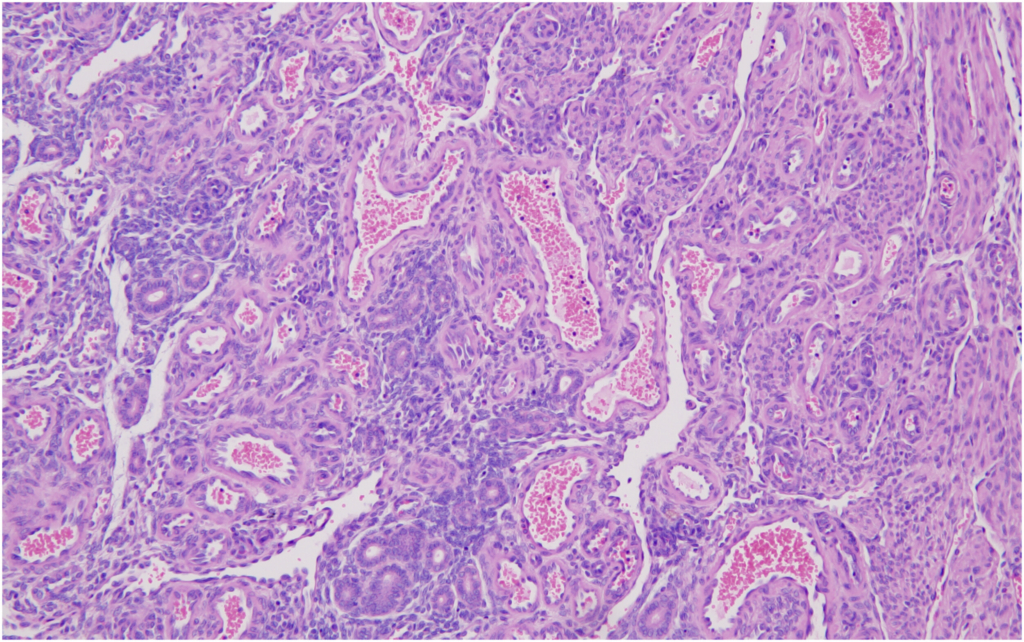
Bronchiolo-alveolar adenomas of the lungs were the most frequently observed spontaneous neoplasms in both male and female mice in the present study, with incidences of 10% in males and 8% in females. These findings are consistent with previously published data by Paranjpe et al,
7 which reported incidences of 11.69% and 8.51% in male mice and 6.47% and 6.35% in female mice across two study periods.
In comparison, Labcorp
16 reported lower incidences of bronchiolo-alveolar adenomas in males (6.3%) and females (2.3%), suggesting a reduced background occurrence in their data relative to our findings.
In our study, bronchiolo-alveolar adenocarcinoma was observed in 4% of male mice and was absent in female mice. These findings were compared with historical data from Paranjpe et al and Labcorp. Paranjpe et al
7 reported lower incidences in male mice of 0.56% and 2.21% and higher incidences in female mice of 1.27% and 2.00% across the two study periods. The data from Labcorp showed an incidence of 1.6% in male mice, which was lower than our findings, and 0.5% in female mice,
16 which was consistent with our results.
Overall, the incidence of bronchiolo-alveolar adenocarcinomas was notably lower than that of bronchiolo-alveolar adenomas across all datasets, including the current study.
The combined incidence of bronchiolo-alveolar adenomas and adenocarcinomas in the lungs was 14% in male mice and 8% in female mice in the present study. This finding of total lung tumors is consistent with the data reported by Paranjpe et al,
7 which documented combined incidences of 12.25% and 10.72% in male mice and 7.75% and 8.35% in female mice across the two study periods. In contrast, Labcorp reported lower combined incidences of 7.9% in males and 2.8% in females, suggesting a reduced background rate of spontaneous neoplastic findings of lungs in their studies.
16The incidence of bronchiolo-alveolar adenomas of the lungs (10% in males and 8% in females) was also compared with other published data wherein the earlier data from CIEA
18 reported slightly lower incidences of 5% in males and 6% in females, while more recent data from CLEA
18 showed higher rates of 11.6% in males and 11.1% in females, closely aligning with our observations.
In contrast, bronchiolo-alveolar adenocarcinomas were less frequent. The current study reported an incidence of 4% in males, which was lower than the 7% observed in Central Institute for Experimental Animals (CIEA)
17 data and absent in the Central Laboratory for Experimental Animals (CLEA) dataset. Similarly, in females there was no incidence of bronchiolo-alveolar adenocarcinomas in the current study which was in close alignment of 0.6% incidence as observed in the CLEA,
18 while the CIEA data reported an incidence of 3% in female mice.
When considering the combined incidence of lung tumors (adenomas and adenocarcinomas), the present study reported 14%, which aligns closely with CIEA at 12% and CLEA at 11.6%. This overall consistency supports the reliability of lung tumor data across all these studies.
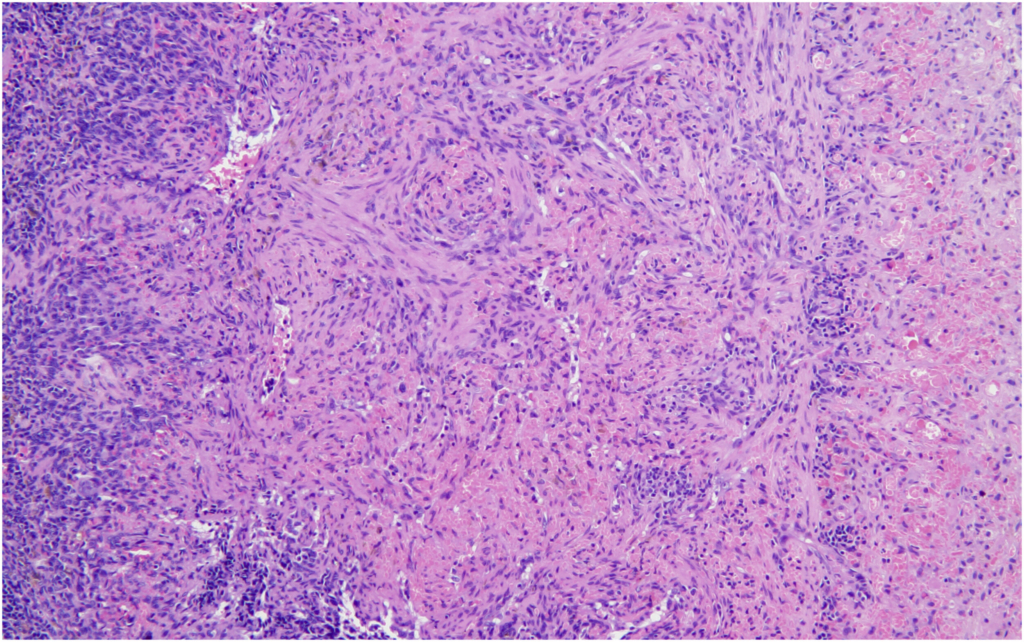
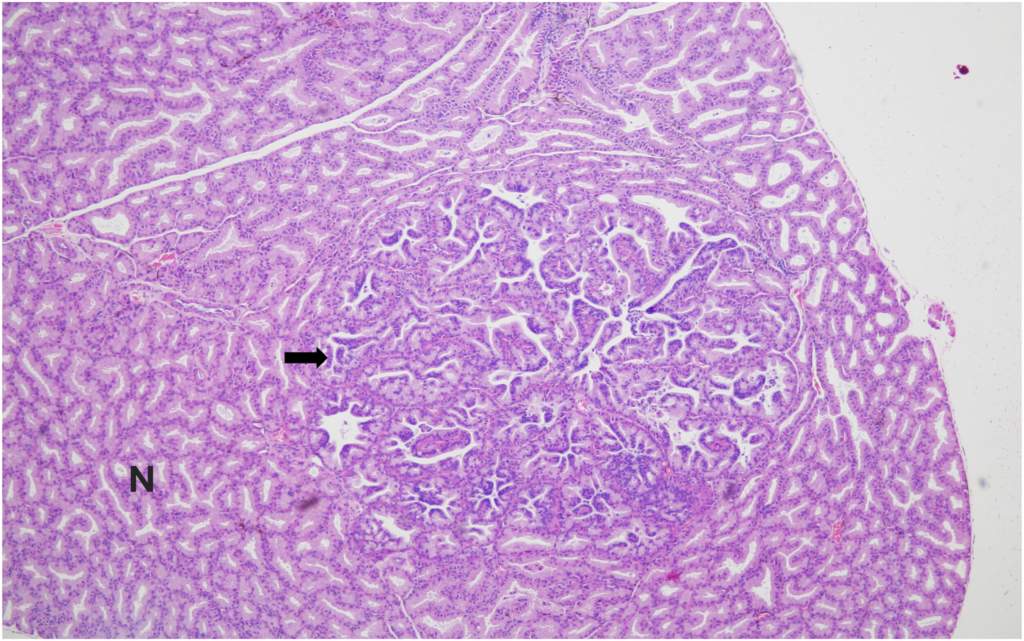
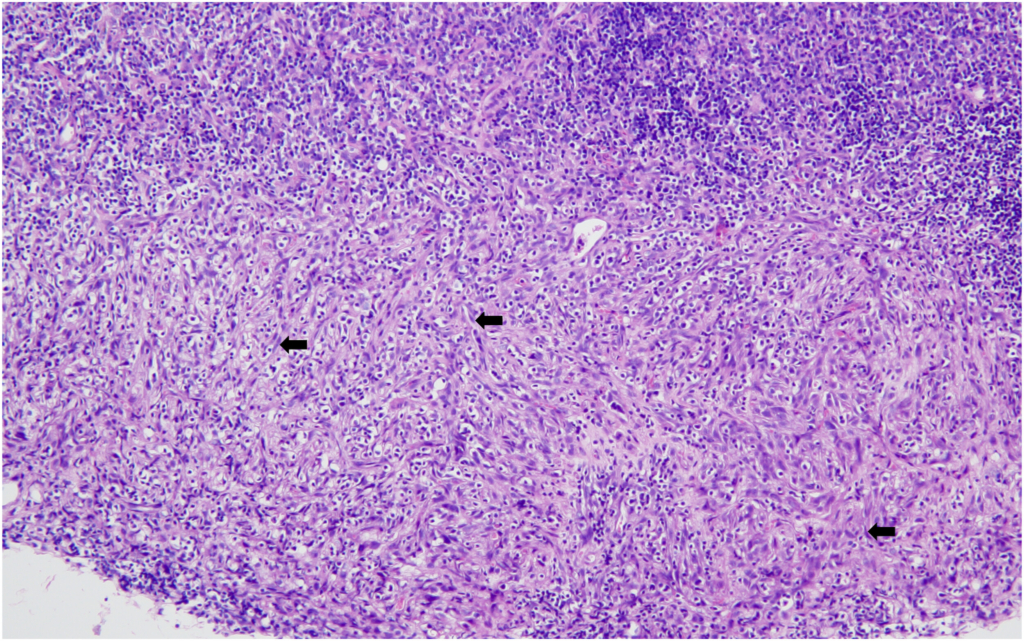
Hemangiosarcoma of the spleen was identified as the second most common spontaneous neoplasm in both male and female mice in the present study. The incidence was 2% in male mice and 4% in female mice, aligning closely with previously reported data. Historical data from Paranjpe et al documented slightly higher incidences of 3.66% and 3.87% in male mice and 3.66% and 3.76% in female mice, respectively. Similarly, Labcorp reported incidences of 3.8% in males and 3.5% in females, further supporting the consistency of this lesion across studies.
The incidence of splenic hemangiosarcoma when compared with other data from mice sourced from Japan revealed that the historical data from CIEA reported higher incidences of 8% in males and 7% in females, while more recent data from CLEA showed an incidence of 4.4% in males and 6% in female mice.
These comparisons suggest that while the incidence of splenic hemangiosarcoma was less in our study (2 % males and 4% in females), it remains a reproducible and prominent background neoplastic lesion in these mice.
It is known that Tg.rasH2 mice also occasionally develop hemangiomas and hemangiosarcomas in organs other than the spleen. These tumors present grossly as blood-filled nodules or masses. The microscopic features of these hemangiosarcomas are very similar to the ones described for the spleen above. No hemangiomas were observed in any of the mice. Hemangiosarcoma was also observed in the testes/uterus, with an incidence of 2% each, while its average incidence in the referred publications was found to be lesser in males (0.7% and 0.22%, respectively) and somewhat variable in females (1.41% and 0.12%, respectively). However, it is worth noting that the two publications of Paranjpe et al also describe the “range” of % incidence of all tumors in the studies they used for creating their historical database, and the % range per study for hemangiosarcoma in testes was reported to be 0%–4% while that in the uterus was 0%–8% and 0%–4%.
5 Hemangiosarcoma in the testes was also reported to be 1.1% while that in the uterus it was 2% as reported by the CLEA.
18 Hemangiosarcoma in the testes and uterus was not reported by Labcorp.
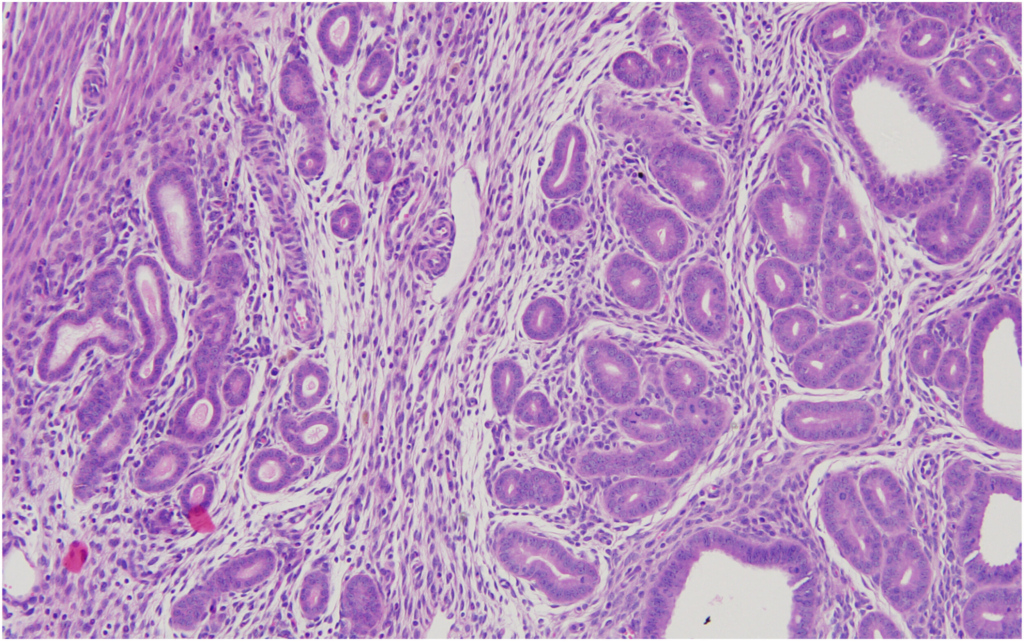
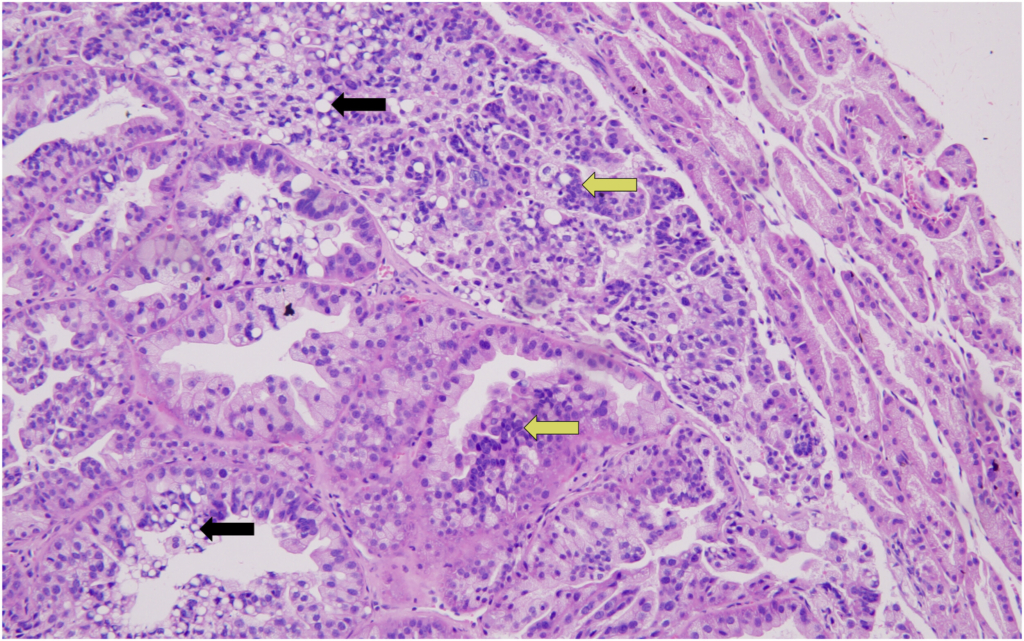
16Accordingly, the incidence of adenocarcinoma of the Harderian gland in male mice in our study was 2%, while its average incidence was 0.14% and 1.44%, respectively, in the two publications and had a range of 0%–4% and 0%–12%, respectively.
5 Similarly, the reported incidence of the adenocarcinoma of the Harderian gland in male mice was 0.3% and 5%, respectively, as reported by Labcorp and CIEA.
Although there was no incidence of adenoma of the Harderian gland in male mice in our study, its average was 1.41% and 1.33%, respectively, in the two publications, with a range of 0%–8%. The adenoma of the Harderian gland in female mice had a 2% incidence in our study, and it was comparable with the 2.81% and 1.29% average incidence of this tumor in the two publications (range, 0%–16% and 0%–8%), respectively.
5 The incidence of adenoma of the Harderian gland was 0.7% and 1.4% in male and female mice, respectively, as reported by Labcorp.
16Similarly, the reported incidence of the adenoma of the Harderian gland in female mice was 1% as reported by the CIEA.
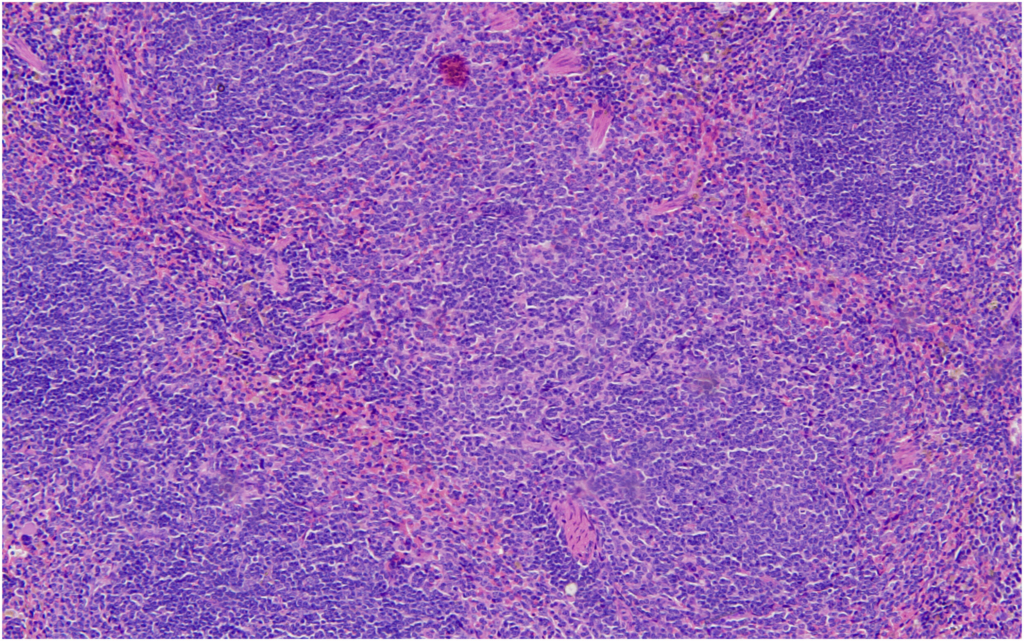
17Likewise, our study revealed a 2% incidence of thymoma in female mice, as compared to the 0.56% and 1.06% average incidence in the two publications by Paranjpe et al, respectively (range, 0%–12%). Comparative data from Labcorp indicated a thymoma incidence of 0.7% in male mice and 2.2% in female mice, placing our findings in close agreement with their female cohort.
In our study, the presence of squamous cell carcinoma of the salivary glands was noted only in one female mouse (incidence of 2%). The corresponding average incidence of this tumor in female mice, as cited by Paranjpe et al, was 0.14% and 0.35%, respectively (range, 0%–4%). The absence of this tumor in male mice in our study was in agreement with the one of the databases of Paranjpe et al,
7 while their other database reported a single male mouse with this tumor (average incidence of 0.14%) when compared across two study periods. Similarly, squamous cell carcinoma of the salivary gland either in male or female mice was not reported in the data published by Labcorp, CLEA, and CIEA.
However, there were a few other tumors that were observed only in the present study conducted at Intox on the mice sourced from CLEA, Japan, and were missing from the two historical control databases from the mice sourced from Taconic, USA, or as reported by other authors in their published data. These included one keratoacanthoma in skin of the head (females) and one rhabdomyoma of the skeletal muscles.
Above discussions reveal that despite the variables such as the wide range of the study conducted dates by Paranjpe et al,
7 or by Labcorp,
16 geographical locations, number of mice in study, rodent feed, and husbandry practices across the institutions, the tumor spectrum in our study was remarkably similar to these studies. The common spontaneous tumors noted in rasH2 mice sourced from Taconic Inc., USA, were pulmonary adenomas/carcinomas, splenic hemangiosarcomas, and Harderian gland adenomas/carcinomas. Two exceptions noted in our study were the isolated instances of keratoacanthoma of skin and rhabdomyoma in skeletal muscles. The similarity of the incidence of the spontaneous tumors as observed predominantly in the lungs and spleen in this study and when compared with a large database collected over a long-time interval also demonstrate the robustness and minimal drift in this animal model.
The tumors observed in urethane-treated male and female mice in this study composed of 100% incidence of bronchiolo-alveolar adenomas and 70% incidence of bronchiolo-alveolar adenocarcinomas in lungs (males, 30%; females, 40%), while there was 100% incidence of hemangiosarcomas in the spleen. Similar observations were made in published studies that used urethane as the positive control, which showed a 100% incidence of bronchiolo-alveolar adenomas in Tg.rasH2 mice and adenocarcinomas (males, 30%; females 22.22%) and splenic hemangiomas (20% in males only)/hemangiosarcomas (males, 80%; females 60%). These findings thus validated the use of urethane as a positive control agent in Tg.rasH2 mice, as an alternative to MNU.
8 Back to News
Back to News