One of the major issues encountered during drug discovery and development is compound’s low aqueous solubility resulting in low bioavailability and developability issues. Nanosizing of poorly soluble drugs is one of the most promising approaches employed, besides other approaches, to enhance bioavailability and to enable compounds to toxicity studies. Nanosuspensions have unique advantages in terms of its application for wide range of molecules, low excipient/vehicle concentration and ease of usage/administration.
Nanosuspensions are colloidal dispersions containing drug particles uniformly dispersed in an aqueous vehicle containing wetting agents and stabilisers with typical particle size below <1µm, preferably <0.5µm. The basic principle of this technique involves reduction of particle size to submicron range thereby increasing the surface area by several folds enabling faster dissolution rate. The particle size reduction is accomplished by right selection of wetting agents, stabilisers, and optimising ball milling process parameters.
In this case study, we would explain how Aragen’s formulation scientists devised a formulation strategy and protocol to develop a robust nanosuspension formulation for a poorly soluble compound to achieve the desired bioavailability and in vivo exposure.
The client is a biotech company based in the USA and focused on drug discovery and development. The project goal was to develop orally bioavailable nanosuspension formulation for a poorly soluble compound using Aragen’s proprietary protocol and technology. The compound is a crystalline material with a melting point of 199o C and solubility in FaSSIF of 6µg/mL and oral bioavailability less than 5%.
Aragen devised a systematic screening and development approach, over a period of 4 weeks, to identify suitable polymer and surfactant to obtain a stable, monodisperse nanosuspension using ball milling process.

Step 1: Polymer Screening: (8 trials – 50mg per trial, Duration – 1 week)
The screening involved 8 polymers and 1 surfactant with 50mg compound in 5 mL per trial. The compound was suspended in an aqueous solution containing polymer and surfactant and micronized using a probe sonicator to reduce particle size to micron range. The suspension was milled using a ball mill with 0.5mm ZrO2 balls for 60 minutes. The resulting nanosuspension was characterised for particle size distribution (PSD), PXRD, SEM, PLM, Assay and Purity. The best polymers were selected for next stage based on the characterization data.
Figure 1 demonstrates the dynamic light scattering data of the nanosuspension, indicating monodisperse distribution of the suspension & Figure 2 shows the image of Drug and it’s Nanosuspension under Polarized light microscopy (PLM).

Step 2: Polymer & Surfactant Screening: (15-20 trials – 50mg per trial, Duration – 1 week)
The screening involved 2-3 best polymers selected from the step-1 screening and 8 surfactants with 50mg compound in 5 mL per trial. The screening was conducted using the same process outlined in step-1 screening. The best polymer and surfactant combinations were selected for the next stage based on the characterization data. Optimization Parameters Suspension Media (polymer + surfactant) Bead & sample fill volume Wet milling time Let’s begin the Conversation E: bd@aragen.com W: aragen.com /company/aragen-life-sciences /AragenLifeSciences India • USA • Netherlands • Japan • Italy • S Korea
Step 3: Drug load optimization: (5-10 trials – 50mg per trial, Duration – 1 week)
The screening involved 2-3 best formulations selected from step-2 screening and evaluating drug concentration up to 50mg/mL using same process outlined in step-1 screening. The best formulations were selected for scaleup and stability studies based on the characterization data.
Step 4: Scale up and Stability studies: (2-3 trials – 500mg per trial, Duration – 2 weeks)
Scale up batches were prepared for selected formulations and loaded for stability studies at 2-8o C, 25o C/60%RH, 40o C/75%RH for 4 weeks. The batches were characterized for particle size distribution (PSD), PXRD, SEM, PLM, Assay and Purity. One formulation was selected for in vivo PK study based on the 1-week stability data followed by execution of PK study in rat.
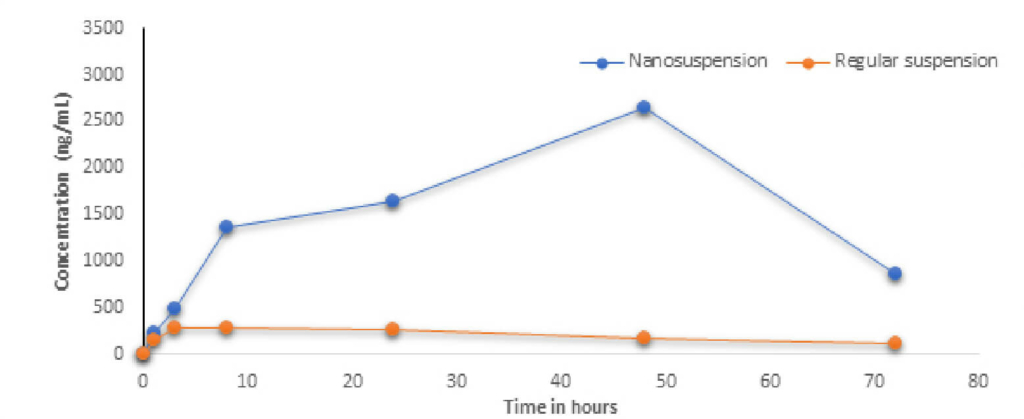
A stable nanosuspension was successfully developed at drug concentration of 50 mg/mL with particle size of d90 <0.5µm. The suspension was physically and chemically stable for 4 weeks. In vivo study demonstrated that nanosuspension significantly improved the bioavailability & plasma concentration compared to microsuspension by several folds paving the way for further development of the molecule with clear formulation strategy to support Toxicity and Clinical studies.