

Oral drug delivery is common, but over 40% of emerging new chemical entities (NCEs) have poor aqueous solubility and low permeability, leading to suboptimal results. Microemulsion formulations significantly enhance aqueous solubility of the water-insoluble drug, facilitating effective drug incorporation. Additionally, they improve bioavailability, ensure stability, prevent drug precipitation, and enable controlled and sustained drug release for better therapeutic outcomes with reduced dosing frequency.
Here we report, one of the projects executed for a US based client where the formulation scientists of Aragen developed a robust and scalable methodology for developing microemulsion formulation for a water insoluble drug. The protocol entails extensive and meticulous optimization of experimental parameters enabling physicochemical stability of the microemulsion. Additionally, efficient resource optimization was carried out for selection of appropriate excipients and identification of appropriate parameters for precise and reliable analytical characterization of the formulation. The protocol also involved pre-clinical evaluation of the formulation in male beagle dogs to access the absorption and biodistribution of the optimal formulation.
The client wanted to develop a water-based microemulsion, characterized by extremely small, emulsified droplets for a lipophilic drug. Aragen was approached, considering its expertise in developing range of formulations including microemulsions. The key objective of the project was to develop a thermodynamically stable microemulsion, capable of maintaining stability across a broad temperature spectrum, owing to its exceptionally fine droplet size, typically falling within the range of 0.01 to 0.05 μm. Furthermore, the objective was to also improve the solubility of the lipophilic drug in an aqueous environment and enhance its permeability through intestinal efflux barriers.
The client is a specialized company focused on the development and advancement of precision therapies for novel anti-cancer and antiviral pharmaceuticals, designed for the treatment of late-stage cancers and chronic viral infections.
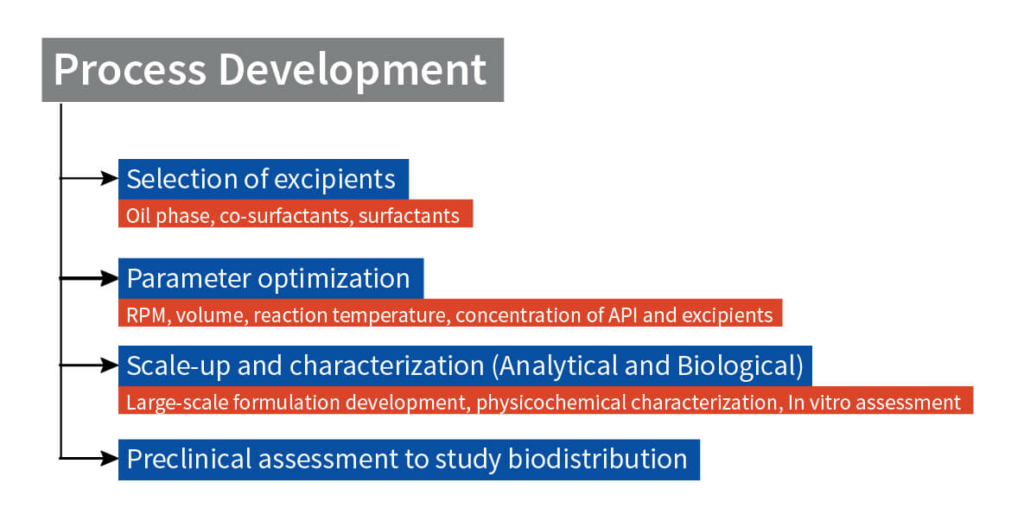
The project initiated with excipient selection (Oil, Surfactant, and Co-surfactant) and identification of the appropriate ones by developing numerous microemulsion formulations at wide range of physicochemical experimental parameters. The ideal excipients and the appropriate experimental parameters were identified following the analytical characterization of the formulations as accelerated conditions. Formulations exhibiting thermodynamic stability over a wide temperature range were characterized for their biological activity using in vitro assays in numerous cell lines and preclinically tested in male beagle dogs for studying the biodistribution in blood plasma to establish its efficacy and safety profile.
Step 1: Selection of excipients
Step 2: Preparation and Characterization of Microemulsion
Predetermined amounts of the drug were dissolved in the required quantity of oil phase. Followed by addition of surfactant and co-surfactant at a fixed ratio. Distilled water was added gradually with continuous stirring, which resulted in the formulation of a transparent and homogenous microemulsion. Further various scale up batches were prepared with optimized excipients concentration and loaded at accelerated condition for short- and long-term stability assessment. At every step of the process development all the formulations were characterized for physicochemical and biological properties.
40 mg drug per trial at 20 mg/mL, Duration: 4-5 weeks
The developed microemulsion formulation enhanced the solubility and oral bioavailability of poorly water-soluble drug. The stability studies confirmed that the optimized microemulsion was stable physically and chemically at short term accelerated conditions. Results from the in vivo studies revealed that the developed microemulsion formulation possessed a 15-fold increase in absorption, compared to the plain drug solution.


