

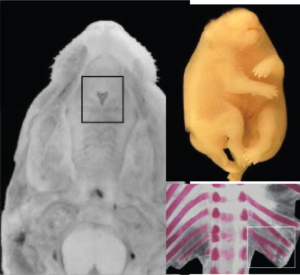
Examples of Single Staining
(Alizarin Red S stain):
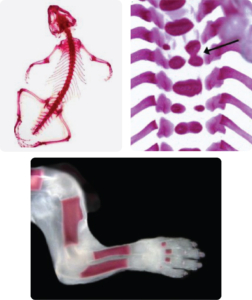
Examples of Double Staining
(Alizarin Red S and Alcian Blue stain):

Sperm motility and Morphology Analysis (SMAS) System is an advanced Japanese CASA System for Precise Evaluation of Sperm Quality. With its user-friendly interface, high-resolution analysis, and an innovative sperm identification algorithm, SMAS accurately measures motility, morphology, and concentration of human and animal sperm. Equipped with a 5-megapixel camera, it eliminates measurement errors during semen examination, offering a remarkable edge in assessing spermatozoa.

| Study Title | Guideline Followed |
|---|---|
| Prenatal and Postnatal Developmental Toxicity Study in Rats with Assessment of Immunogenicity and Bio-distribution (Covid DNA Vaccine) |
WHO Technical Report Series, No. 927, 2005 |
| Prenatal and Postnatal Developmental Toxicity Study in Rat (TdaP2 Vaccine) |
WHO Technical Report Series, No. 927, 2005 |
| Prenatal Developmental Toxicity Rabies Vaccine (inactivated) and H1N1 inactivated / attenuated Vaccine |
OECD/ICH/WHO |
| Prenatal Developmental Toxicity of EX-Vivo cultured human mesenchymal stem cell in Rat |
OECD/ICH/WHO |
| Reproduction / Developmental Toxicity Screening Test of Implant mammaire monobloc® Silicone SoftOne®-HP (Haut Profil) 265 MT (micro texture) in Wistar Rat |
OECD No. 421, ISO10993-Part 3 and ISO10993 Part 12 |
Intox Pvt Ltd. is a wholly owned subsidiary of Aragen Life Sciences, a leading R&D and manufacturing solutions provider, for the global life sciences industries offering integrated or standalone solutions for small and large molecules. Its OECD GLP-certified and AAALAC-accredited test facility has conducted over 15000 GLP studies and has a successful track record of data submission to global regulatory authorities in USA, Canada, EU, UK, Brazil, Argentina, Japan, India, Australia, and China.

