

Due to extensive immuno-oncology (IO) research, more effective and targeted treatment strategies for cancer have evolved in recent years. The approval and use of chimeric antigen receptor (CAR) T-Cell therapeutics in difficult-to-treat cancers has brought hope to millions of patients and oncologists globally. In particular, the success of CART-cell therapy in hematologic malignancies renewed efforts to use this technology against solid tumors as well. However, this strategy has encountered several challenges, including targeted delivery, penetration of therapeutics to tumor cells, immunogenicity, short- and long-term toxicity and safety, and efficacy of CART-cells in humans. To address the challenges above, several animal models have been utilized to screen potential CART-cell therapeutics as part of the IND-enabling process.
In addition, bispecific antibodies have emerged as a new class of therapeutic agents designed to simultaneously bind to patient’s T cells and to tumor cells, inducing T-cell-mediated cytotoxicity of tumor cells. Humanized models with human peripheral mononuclear blood cells (PBMCs) in combination with a variety of xenograft models, are the most frequently used in-vivo platform for short-term screening of novel compounds. Full hematopoietic stem cell (CD34+) reconstituted models combined with genetically modified immunodeficient strains are utilized for long term screening of T-cell modulators, natural killer (NK) cell modulators, and other agents that induce antibody dependent cell cytotoxicity (ADCC), as well as various categories of immune checkpoint inhibitors and agonists.
Aragen Life Sciences is a leading R&D and manufacturing solutions provider for the life sciences industries worldwide offering end-to-end integrated and standalone solutions to advance small and large molecule programs from concept to commercialization. In vivo pharmacology services at Aragen Bioscience offer specialized, customized, comprehensive portfolio of in vivo services for testing of your investigative immune-oncology cell therapeutics.
A team of experienced scientists and skilled in vivo research associates can support pre-clinical research from study design through execution as follows:
Our research facilities are in Morgan Hill, CA and we can accommodate same-day or second-day domestic shipping of your temperature-sensitive therapeutics and biological samples.
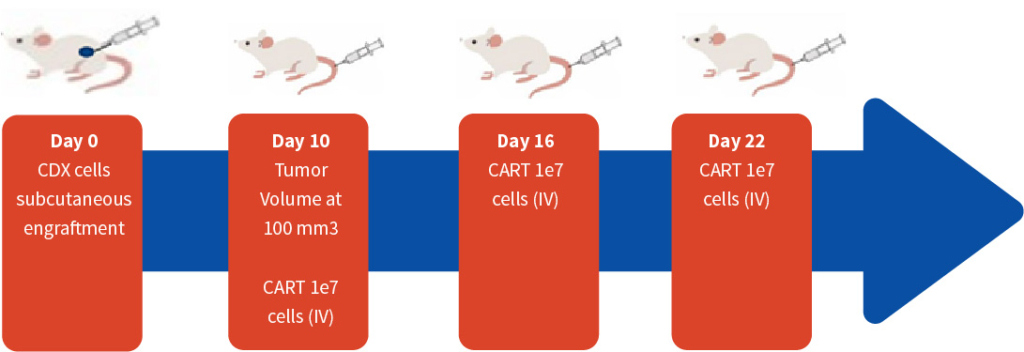
Figure 1. Representative study design for evaluating CART therapeutics
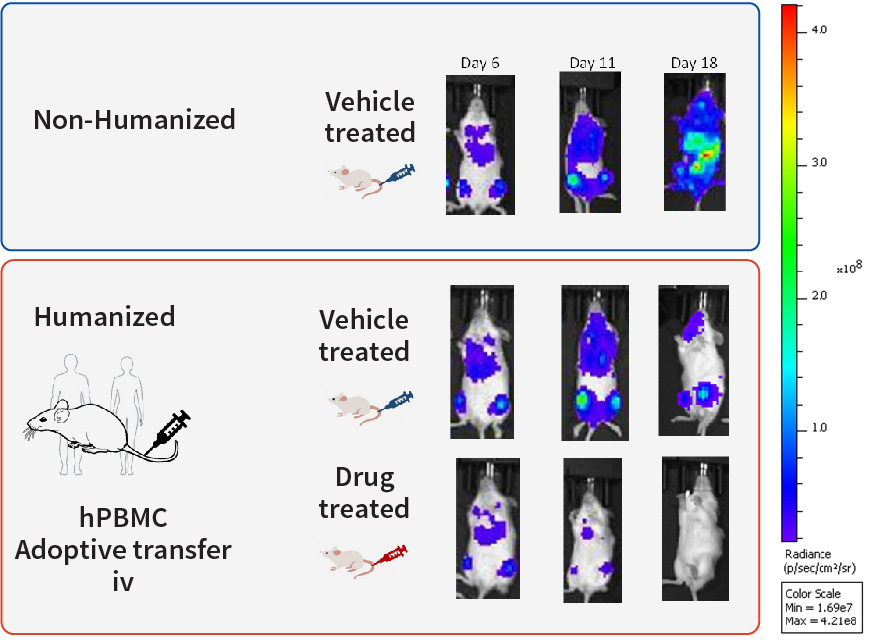
Figure 2. Ventral view images of mice after inoculation (6, 11 and 18 days) of Raji-Fluc cells both in non-humanized and humanized models.
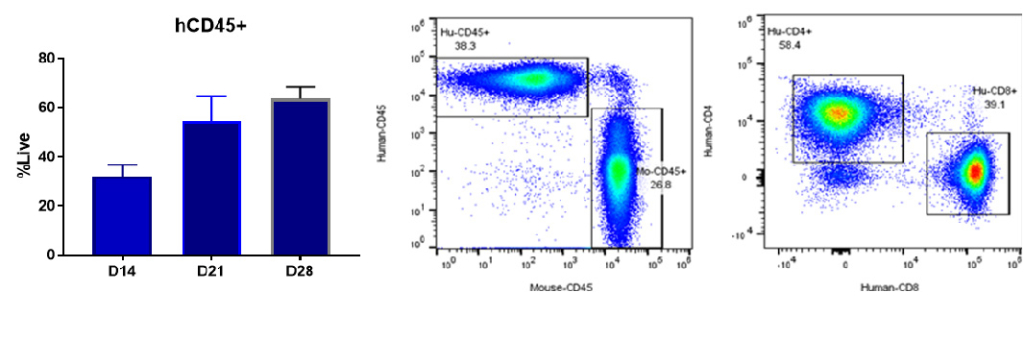 Figure 3. Engraftment of human PBMCs
Figure 3. Engraftment of human PBMCs
Bar graphs of CD45+ engraftment after hPBMC adoptive transfer. The leukocyte marker CD45+ and T-cell markers CD4+ and CD8+ measured from peripheral blood on 14, 18 and 21 days.
Harbani K. Malik-Chaudhry, (2021) TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL, mAbs, 13:1, DOI: 10.1080/19420862.2021.1890411.

