

Recombinant proteins (RP), produced through genetic engineering, include a diverse range of proteins including antibodies, enzymes, and vaccines, widely used in the biopharmaceutical industry. These proteins are successfully produced with E. coli-based expression systems that offer rapid, scalable, and cost-effective synthesis of RPs. However, inherent challenges can impede efficient protein production, leading to low yields.
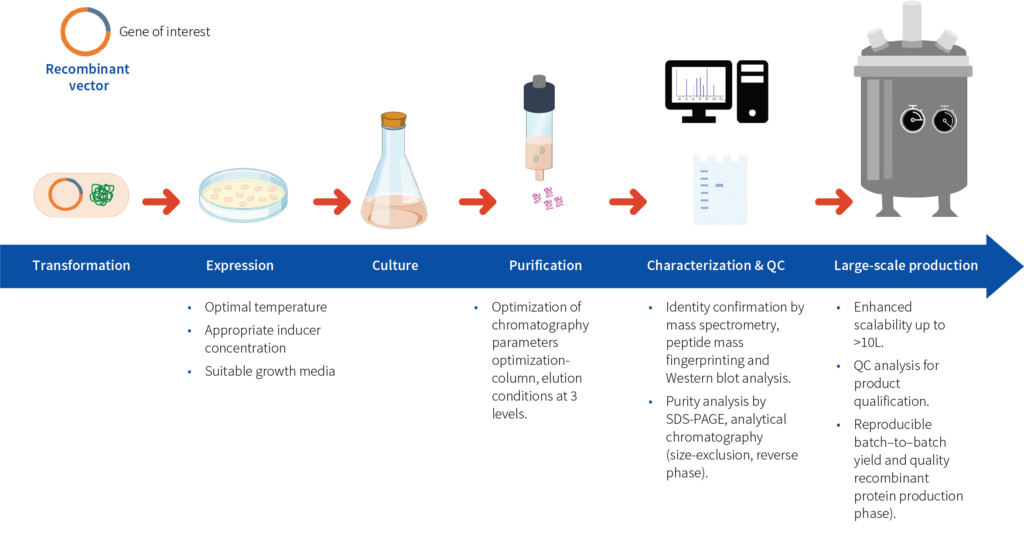
At Aragen, our experienced scientists offer a streamlined protein expression and purification process (Figure 1) that helps in overcoming the challenges encountered with E. coli protein expression systems through effective mitigation strategies to optimize yield, quality, and functionality.
Improper protein folding and solubility: Recombinant protein expression in E. coli often encounters hurdles primarily related to protein folding, which directly impacts protein solubility and functionality.
Mitigation strategies:
Lack of specific protein folding conditions: Some proteins require post-translational modifications or specific folding conditions that are absent in the E. coli cytoplasm.
Mitigation strategies:
Codon Bias: Preferential usage of the target gene’s synonymous codons causes inefficient expression in E. coli expression systems.
Mitigation strategies:
Protein Degradation: E. coli contains proteases that might degrade recombinant proteins, reducing overall yield and purity.
Mitigation strategies:
Toxicity: Some recombinant proteins may be toxic to E. coli cells, leading to poor growth or low protein expression levels.
Mitigation strategies:
Outcome: Maximized soluble protein expression in E. coli system, reduced production costs by 20-30% (reduction in both FTE hours and material cost) with a substantial drop in turn-around time by 20%.
At Aragen, we have over 10 years extensive experience in the field of recombinant protein production. Our clientele comprises biopharma and biotechnology companies focused on protein engineering and targeting to create multifunctional, precision therapies for various indications such as cancer, hematology, immunology, and rare diseases. We also extend our services to animal health and agroscience companies.
Ready to elevate your game in the recombinant protein industry? Write to us at info@aragen.com to speak to our expert.

